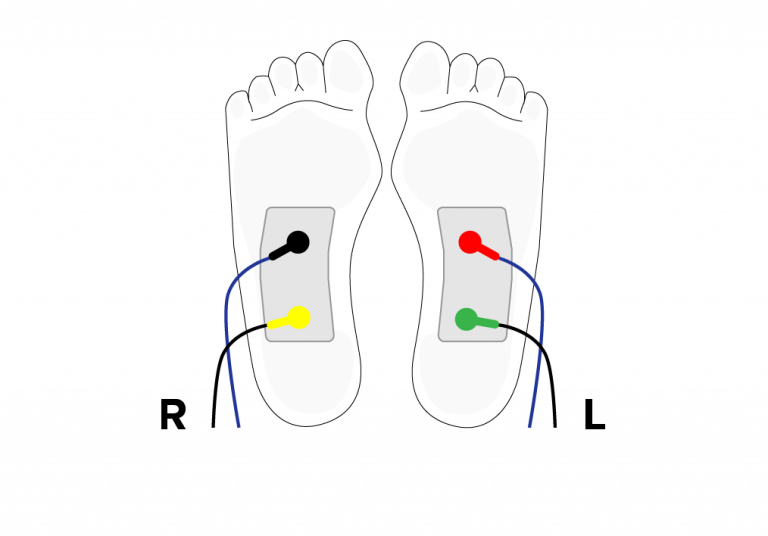
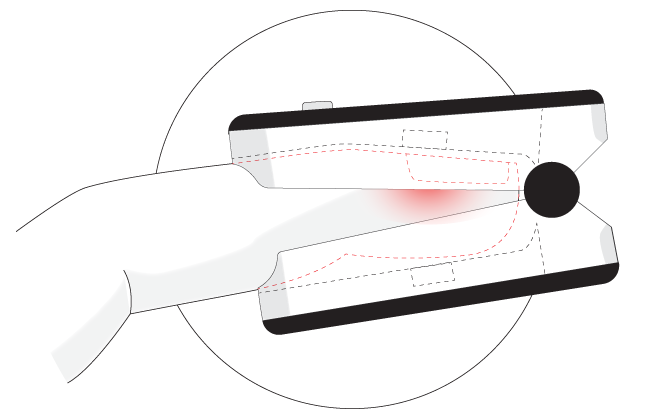
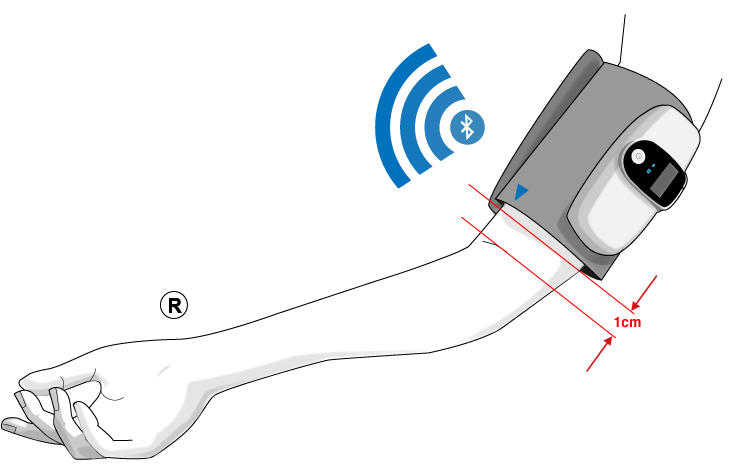
Express delivery and returns within 24 hours
Office:
+1 (305) 927-2379
100 N. Biscayne Blvd Suite 1903 Miami Florida 33132
Miami, Florida
Mon - Fri:
9:00 am - 5:00 pm